
Aspivix receives U.S. FDA 510(k) Clearance for Carevix™, Their Novel Cervical Stabilizer
Renens, Switzerland – 26 January 2023 – ASPIVIX SA, an innovator and developer of medical technologies to advance gynecological care, today announced that Carevix™, its novel Atraumatic Cervical Stabilizer, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
Carevix™ is an atraumatic cervical stabilizer that utilizes a gentle approach to reduce pain and bleeding in multiple transcervical procedures, such as Intrauterine Device insertions. In our ADVANCE Women, single-blinded, randomized, multicentric, comparative study of 100 women who underwent an IUD insertion with either the Carevix™ device or a traditional cervical tenaculum, women reported statistically significant results with up to 73% reduction of pain scores and 78% reduction of bleeding occurrences in favour of the Carevix™ device.1
Carevix™ is the result of our constant commitment to make gynecology, now modern.
Mathieu Horras, Chief Executive Officer of ASPIVIX emphasized: “With the 510(k) clearance of Carevix™, a design-award winning device, we will provide our U.S. users with an innovative and easy-to-use system that brings a gentler alternative to a century-old gynecological tool. Extensive research was incorporated into the development of Carevix™ so we know the unique and differentiating features it demonstrates with significantly less pain and bleeding that has the potential to dramatically improve the IUD adoption and placement experience for millions of American women.”
About CarevixTM
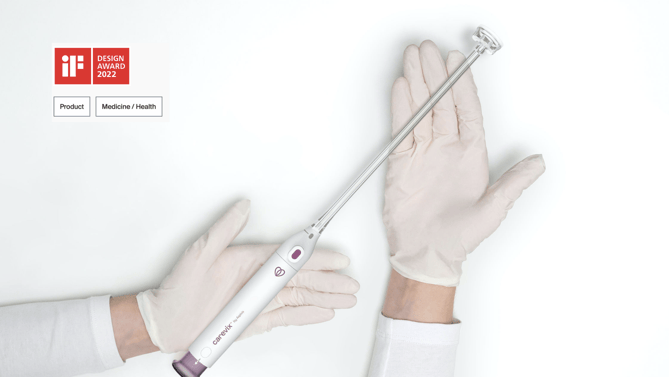
Carevix™ is an innovative, soft-suction cervical device designed as a modern and gentler alternative to a cervical tenaculum when stabilization of the cervix is needed. By leveraging suction technology to stabilize the cervix gently, Carevix™ delivers cervical engagement without the need to perforate the tissue. A semi-circular, anatomical pad is applied to the delicate tissue during gynecological procedures, reducing significantly trauma associated with pain and bleeding.
About ASPIVIX
ASPIVIX SA is a privately held medical device company based in Switzerland, dedicated to developing modern and gentle gynecological solutions that advance women’s healthcare. The Company’s innovative technology development and clinical program are supported by 4FOx Ventures, Zürcher Kantonalbank, HEMEX, Launchpad, Innosuisse, SPEI, EU research and innovation program Horizon 2020, and visionary angel investors.
Visit www.aspivix.com or stay informed www.aspivix.com/stay-informed/
Media Contact:
Mathieu Horras
Mathieu.horras@aspivix.com
+41 79 103 22 06
References
- Dr. Yaron, Michal. “Safety and efficacy of an innovative atraumatic cervical stabilizer for IUD insertion: Results from a randomized, single blind controlled study.” 30th World Congress on Controversies in Obstetrics, Gynecology, and Infertility (COGI), November 24-26, 2022, Amsterdam, The Netherlands. Oral Presentation.
Share this story:










