
ASPIVIX Receives CE Mark Approval for Carevix®
ASPIVIX Receives CE Mark Approval for Carevix® to Reduce Pain During IUD and Transcervical Procedures, Expanding Collaboration with Ambassadors Worldwide and Enhancing Women’s Health Without Fear of Pain.
Epalinges, Switzerland – December 18, 2023 – ASPIVIX, an innovator of medical devices to advance gynecological and fertility care, announced that carevix®, its novel Cervical Stabilizer, has attained CE marking. This next generation device for routine procedures in gynecology will allow millions of women across Europe access to significantly less painful treatments and IUD insertions.

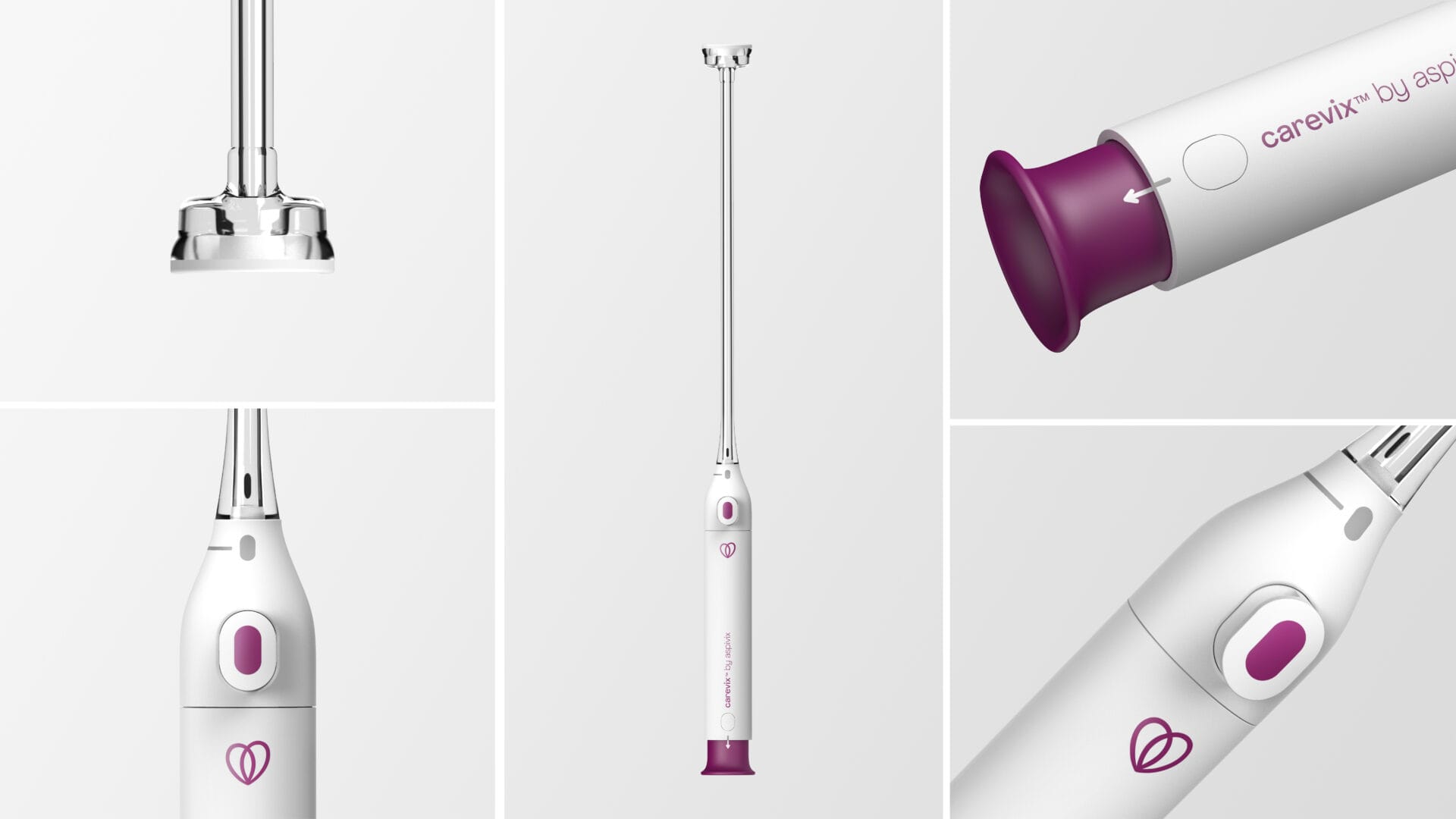
Carevix® is a cervical stabilizer that uses a gentle approach to minimize pain and bleeding during various transcervical procedures, including Intrauterine Device placement. Carevix® is the result of the constant commitment at ASPIVIX to make gynecology, now modern.
Published in the international reproductive health journal Contraception, the results of the ADVANCE Women, a single-blinded, randomized, multicentric, comparative study was conducted on 100 women who underwent an IUD insertion with either the Carevix® device or a traditional cervical tenaculum showed that women who underwent IUD placement with carevix® reported statistically and clinically significant results with up to 73% reduction of pain scores and 78% reduction of bleeding occurrence rates.(1)
The CEO of ASPIVIX, Mathieu Horras, emphasized that “the carevix® device, which had also received 510(k) clearance and won a design award, earlier this year, provides an innovative, clinically proven and user-friendly alternative to a century-old gynecological tool. The extensive research that went into the development of carevix® ensures significant reduction pain and bleeding, improving the IUD adoption and placement experience as well as many other gynecological and fertility procedures for millions of American and European women.”
In addition to the 510(k) clearance, this CE mark approval is a very important milestone that allows ASPIVIX to expand worldwide and to collaborate with Ambassadors (OBGYNs, midwives, nurses) across 12 centers of excellence (USA, France, Sweden, Switzerland, Germany and Austria) using carevix® in routine, gaining invaluable insights and most importantly, providing women a better experience in gynecology. It showcases our commitment to innovation, fostering collaboration, and improving patient outcomes by refining our products in clinical practice.
- CE mark approval follows the US FDA approval from January 2023.
- Pain is the most common reason of avoidance of cost-effective and 99% efficient contraception (IUD), affecting approximately 100 million women globally every year
- Unspoken and underestimated pain leads to delays in diagnostics & treatments, resulting in 121 M unintended pregnancies globally, and 60% of them end in abortion (source: WHO).
About Aspivix
Aspivix is a pioneering leader in women’s health, dedicated to modernizing healthcare solutions for women around the world. With a focus on innovation, Aspivix is committed to developing cutting-edge medical devices, such as Carevix®, which aim to transform the gynecological experience by offering safer and less painful solutions that enhance the quality of care and empower women to take control of their health.
About Carevix®
Carevix® is an innovative, soft-suction cervical device designed as a modern and gentler alternative to a cervical tenaculum when stabilization of the cervix is needed, aiming to significantly reduce trauma associated with pain and bleeding.
Carevix® has been clinically proven to be atraumatic during transcervical procedures. The First-In-Women highly compelling results from the ADVANCE Women (Atraumatic Device using Vacuum Technology for Cervical Procedures in WOMEN), a randomized controlled trial of Carevix®, used In IUD Procedures against the cervical tenaculum has been published in “Contraception”, the International Reproductive Health Journal in March 2023.
Carevix® has received FDA-clearance and now CE-Mark approval in 2023 which will allow Carevix® to be available for commercialization in Europe and the United States of America very soon.
For media inquiries or more information :
Please contact: Ikram Guerd – VP of Global Marketing: ikram.guerd@aspivix.com.
References
(1). Michal Yaron, Hélène Legardeur, Bastien Barcellini, Farida Akhoundova, Patrice Mathevet, Safety and efficacy of a suction cervical stabilizer for IUD insertion: results from a randomized, controlled study, Contraception, 2023, 110004, ISSN 0010-7824, Contraception link
Share this story:










