
The first successful painless IUD and transcervical procedures performed with the Carevix® in Indianapolis, US enhancing Women’s Care in gynecology
Epalinges, Switzerland – March 15, 2024. We are thrilled to announce a groundbreaking achievement in the United States with the first patients benefiting from successful painless IUD placement and transcervical procedures using the non-traumatic, FDA-approved, cervical stabilizer Carevix® aiming to replace the traditional cervical tenaculum.
The 1st patients in the United States with successful procedures
The first painless procedures were successfully performed by Alissa M. Conklin, MD. and Assistant Professor of Clinical Obstetrics and Gynecology at Gynecology at Indiana University School of Medicine.
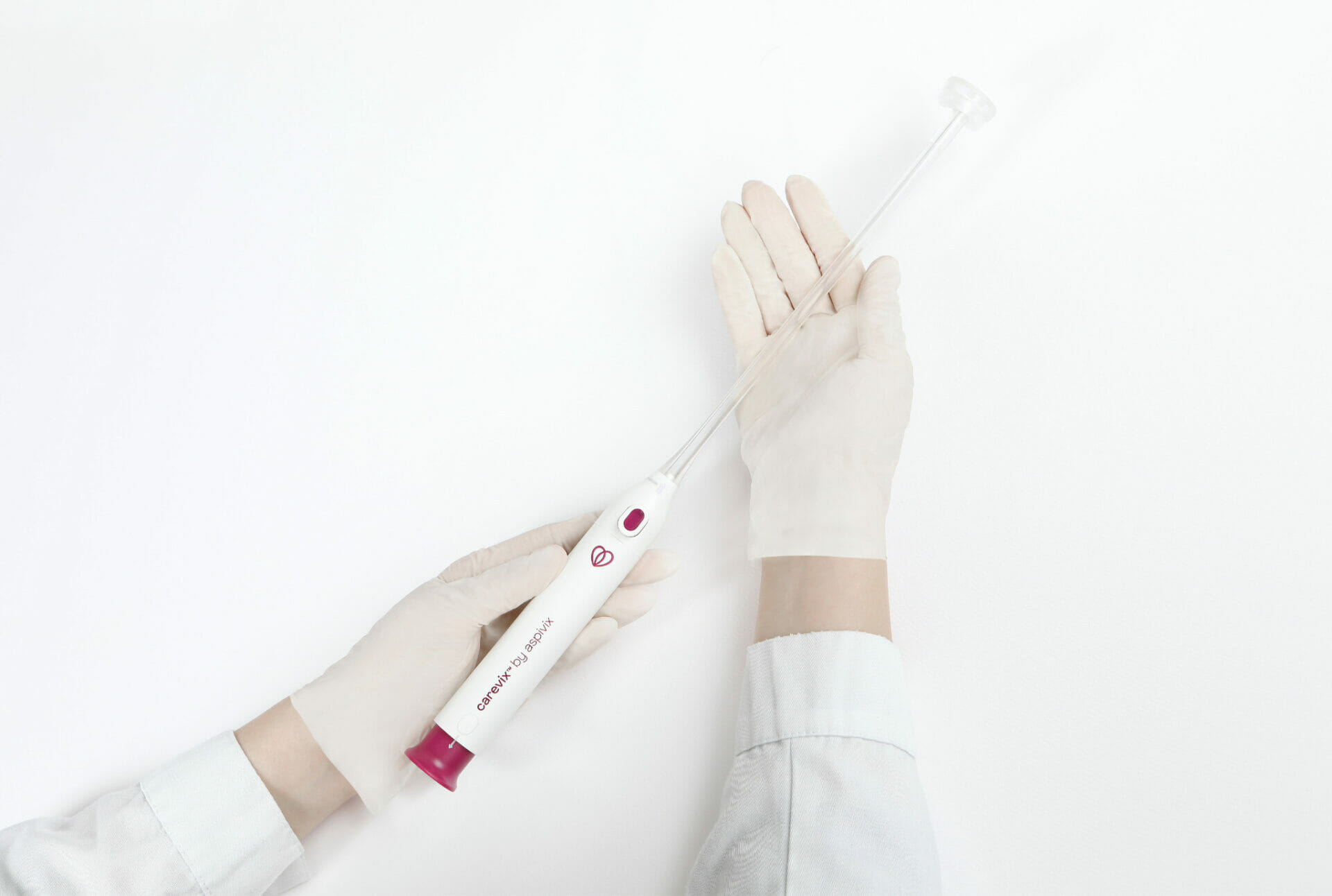
Dr. Conklin said the procedures were conducted with ease and provided excellent suction and traction, allowing adequate access without difficulties. The patient reported significantly less pain and no bleeding compared to previous procedures involving traditional tenaculum use.
“The suction was perfectly adequate and easy to use, even in a non-traditional position! Carevix® allowed us to easily place the IUD when I otherwise could not without cervical traction.” said Dr. Conklin. “When suction was released, there was no bleeding, so we were able to immediately trim the strings and remove the speculum which makes the procedure shorter. The patient had an IUD before and said this device made her experience so much better than it was the last time!“
“It was preferable to prior IUD placement because there was essentially no pain!” shared the first patient satisfied by the procedure that felt more comfortable. “I’m glad research to lessen women’s pain is being conducted!”. Another patient shared “Dr. Conklin also explained that the common tool has been around for a while, so knowing this design of the old tool vs the newer one made me feel like I made the right choice.”
They all felt reassured when presented the device by the healthcare professional.
The Carevix® Ambassador Program allows OB-GYNs, midwives and nurses across 12 centers of excellence worldwide (US, France, Sweden, Switzerland, Germany and Austria), including Indiana to use Carevix® in routine gynecological procedures to provide a better experience for women.
“This program showcases our commitment to innovation, fostering collaboration, and improving patient outcomes by refining our products in clinical practice.” said Mathieu Horras, CEO and Co-Founder of Aspivix.
New clinical study in the United States to improve women’s health
In addition to the Ambassador Program, researchers at IU School of Medicine have consented the first patient in the nation for a clinical study focused on “Safety and efficacy of a suction cervical stabilizer compared to the standard tenaculum for intrauterine procedures in the clinic setting”.
The purpose of this clinical study is to evaluate patient-reported pain, bleeding, and device efficiency along with provider satisfaction and ease of use between intrauterine procedures employing the Carevix® or a single-tooth tenaculum.
This US clinical study complements the Swiss ADVANCE Women study, a single-blinded and randomized study, conducted in the University Hospitals of Geneva that compared the use of Carevix® to the standard cervical tenaculum in 100 women undergoing Intrauterine Device (IUD) placement. The positive results published in Contraception show a significant reduction in pain by up to 73% and in bleeding occurrence by 83% with the Carevix® compared to the single-tooth tenaculum for intrauterine contraceptive device insertion.
This new clinical trial lead by IU School of Medicine broadens the scope of procedures among all intrauterine procedures, not only IUD insertion, including hysteroscopies, sonohysterography and endometrial biopsies.
About Dr. Alissa M. Conklin, MD
Dr. Alissa Conklin is certified by the American Board of Obstetrics and Gynecology, and has dedicated her life to delivering quality and compassionate care to her patients. She is a passionate advocate for patient-centered care with shared decision-making, is recognized for her commitment to trauma-informed care. Graduating from Indiana University School of Medicine Department of OB-GYN in 2012, she has been practicing general obstetrics and gynecology, forming lifelong bonds with her patients. Dr. Conklin believes in empowering individuals through education in the decision making process for their treatment options and to improve their lives, as well as she values a team approach in healthcare.
About IU School of Medicine
IU School of Medicine is the largest medical school in the U.S. and is annually ranked among the top medical schools in the nation by U.S. News & World Report. The school offers high-quality medical education, access to leading medical research and rich campus life in nine Indiana cities, including rural and urban locations consistently recognized for livability. According to the Blue ridge Institute for Medical Research, IU School of Medicine ranks No. 13 in 2023 National Institutes of Health funding among all public medical schools in the country.

About Aspivix
Aspivix is a pioneering leader in women’s health, dedicated to modernizing healthcare solutions for women around the world. With a focus on innovation, Aspivix is committed to developing cutting-edge medical devices, such as Carevix®, which aim to transform the gynecological experience by offering safer and less painful solutions that enhance the quality of care and empower women to take control of their health. For more information, visit aspivix.com.
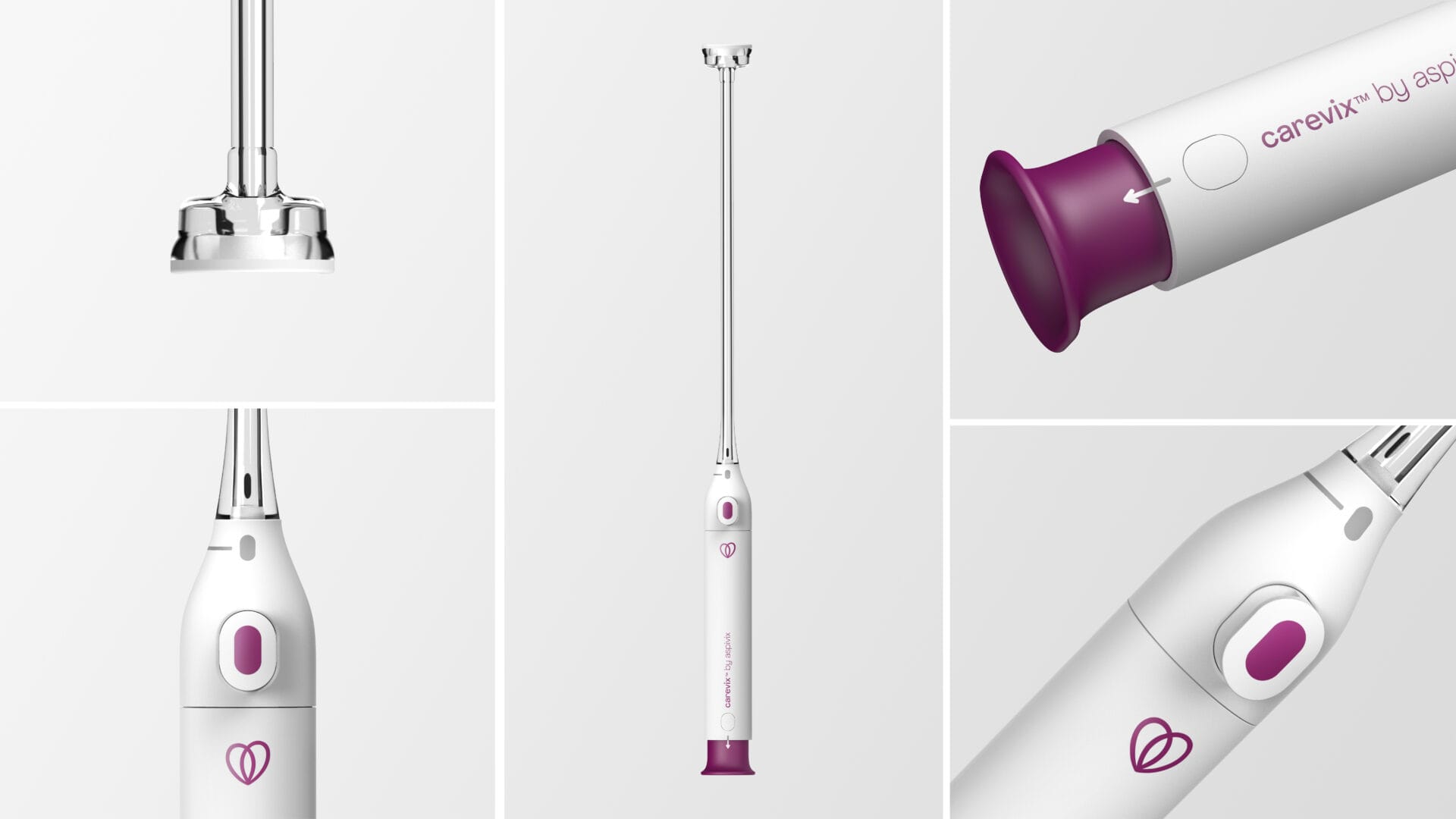
About Carevix®
Carevix® is a novel cervical suction stabilizer that has been clinically proven to be atraumatic during transcervical procedures. The First-In-Women highly compelling results from the ADVANCE Women (Atraumatic Device using VAcuum Technology for CErvical Procedures in WOMEN), a randomized controlled trial of carevix®, used In IUD Procedures against the cervical tenaculum has been published in “Contraception”, the International Reproductive Health Journal in March 2023.
Carevix® has received FDA-clearance and CE-Mark approval in 2023 which allow carevix® to be available for commercialization in Europe and the United States of America.
For media inquiries or more information :
Please contact: Ikram Guerd – VP of Global Marketing: ikram.guerd@aspivix.com.
Share this story:












