The only cervical device
clinically proven to reduce pain during transcervical procedures
Carevix® is a single-use cervical stabilizer, engineered with vacuum technology, fully designed to deliver atraumatic transcervical access
Benefits

Gentle

Intuitive

Versatile

Features
Clinical Evidence
Safety and efficacy of a suction cervical stabilizer for intrauterine contraceptive device insertion: Results from a randomized, controlled study

Study Format
Randomized controlled trial (RCT), comparing the efficacy and safety of Aspivix device, Carevix®, and the standard of care (tenaculum) in intrauterine contraception devices (IUD) procedures.
Primary Endpoint
Patient-reported pain scores (VAS) at specific time points during IUD insertion procedure.
2 Centers in Switzerland
- University Hospital of Lausanne (CHUV)
- University Hospitals of Geneva (HUG)
Clinical Investigators
- Prof Patrice Mathevet, CHUV
- Dr. Hélène Legardeur, CHUV
- Dr. Michal Yaron, HUG
- Dr. Bastien Barcellini, HUG
- Dr. Farida Akhoundova, HUG
Status
Enrollment completed in February 2022
Results
Results published in Contraception, an international reproductive health journal, from the ADVANCE Women (Atraumatic Device using VAcuum Technology for CErvical Procedures in WOMEN), in March 2023.
Results presented at European Society of Contraception in May 2022
Visit PubMed
For clinical studies with Carevix® device, please visit PubMed.
Safety and Efficacy of a Suction Cervical Stabilizer Compared to the Standard Tenaculum for Intrauterine Procedures in the Clinic Setting

Study Format
Interventional study comparing the patient and provider experience and efficiency of Aspivix device, Carevix®, compared to the single-tooth tenaculum for intrauterine contraceptive device insertion (IUD) procedures.
Primary Outcome
Patient-reported during IUD insertion procedure.
Device efficiency with provider-perceived bleeding, provider-perceived ease of use, provider-perceived satisfaction
1 Center in the United States of America
- Indiana University School of Medicine
Clinical Investigators
- Alissa M Conklin, Indiana University (US)
- Pr. Jeffrey Peiper, Indiana University (US)
Status
Enrollment ongoing started in November 2023
Visit ClinicalTrials.gov
Clinical Applications
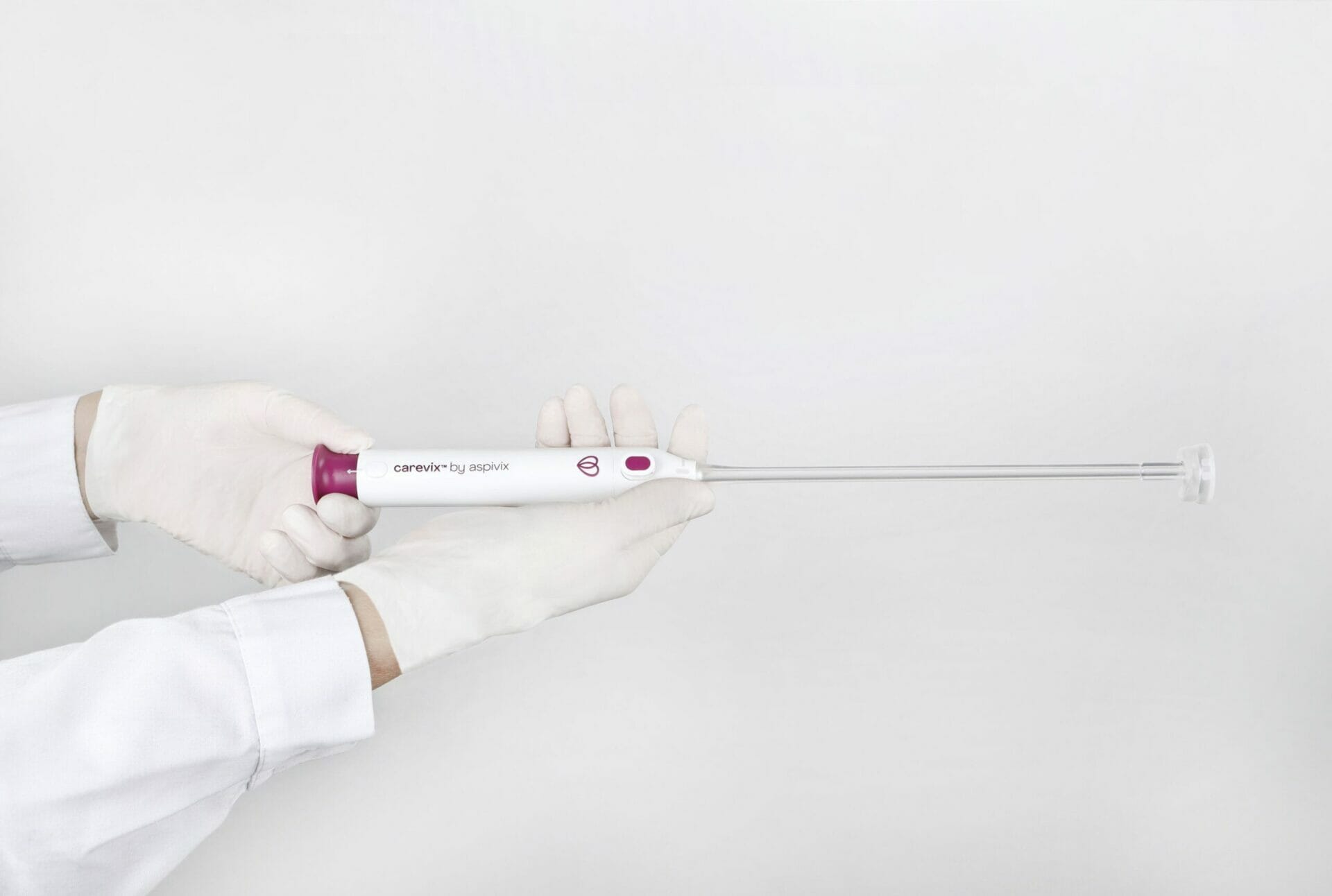
Carevix® is indicated for use in various transcervical procedures, such as:
Are you interested in
being part of the change?
